Chattanooga Continuum Pain Relief Complete System 2600-KIT
Description
Chattanooga Continuum Electrotherapy Pain Relief System
Chattanooga Continuum System is a portable 2-channel stimulator used by therapists in clinics and patients at home to provide electrical stimulation treatments in pain management (TENS) and neuro muscular stimulation (EMS/NMES). By combining TENS with NMES, users can simultaneously help manage pain, enhance exercise and re-train muscles. CAUTION: Federal Law (USA) restricts this device to sale by or on the order of a physician (or licensed practitioner).
Chattanooga Continuum Electrotherapy Pain Relief System Includes
- Chattanooga Continuum device
- Set of 2 pin cables
- User manual and practical guide
- Transportation pouch
- Hand Switch
Why choose the Chattanooga Continuum Kit?
 |
1+1 Function
|
 |
Hand Switch This handheld remote trigger can be used to easily activate stimulation, either by the clinician at a distance, or by the patient |
 |
Generating a strong contraction Continuum delivers muscle stimulation that is powerful whilst remaining comfortable. This allows users to push the intensity of stimulation without the recipient experiencing the sharp spiking sensations of less efficient waveforms. Thanks to this ability to utilise stronger stimulation, it can help the muscle work harder, helping it to grow stronger, faster. |
 |
Kick Stand Keep your hands free by deploying the kick stand during treatment. |
 |
Battery Powered Continuum runs on a pair of standard AA batteries. |
 |
Individually tailorable Continuum has 13 pre-programmed and two custom regimens for NMES and TENS. In addition to being able to control the treatment duration time, the device can modify waveform type (Symmetrical or Asymmetrical), pulse rates and durations (widths), cycling type, off times, channel ramp times, and on time. All of this allows the clinician to customize treatment to help meet the individual needs of each patient. |
 |
Belt Clip The simple belt clip fits over a belt or waistband. |
 |
Heel Switch (SOLD SEPARATELY) When placed inside the user's shoe, this optional heel switch can be used to trigger muscle stimulation, useful for gait training and treatment of drop foot. Please inquire with customer service if you wish to purchase this option. |
When to use Chattanooga Continuum Electrotherapy System?
- This electrotherapy device is great for reeducation and activation of the muscles.
- There are benefits to using the Continuum both before and after an operation for the best therapeutic results.
- It should be used in conjunction with exercise.
- Common conditions to use NMES devices include total knee replacement, total shoulder replacement, rotator cuff surgery, ACL reconstruction, and ankle dorsiflexion weakness.
Benefits of Chattanooga Electrotherapy System
Minimize Shoulder Pain
Rehabilitation therapies after rotator cuff surgery using NMES and TENS, coupled with exercise, help to minimize pain, increase local circulation, and restore flexibility - enhancing recovery.
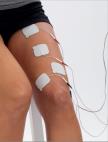
Knee
Reeducate weakened muscle as a result of disuse atrophy
Stroke Gait Control
Continuum's optional heel switch can be used for therapeutic gait training, triggering stimulation of the muscle during contact.
CA Residents Prop 65
WARNING: CANCER & REPRODUCTIVE HARM; PROP 65 WARNING INFO
Proposition 65 requires businesses to provide warnings to Californians about significant exposures to chemicals that cause cancer, birth defects or other reproductive harm. These chemicals can be in the products that Californians purchase, in their homes or workplaces, or th??at are released into the environment. By requiring that this information be provided, Proposition 65 enables Californians to make informed decisions about their exposures to these chemicals.
Are any businesses exempt from Proposition 65’s requirements?
YES. Businesses with less than 10 employees and government agencies are exempt from Proposition 65’s warning requirements and prohibition on discharges into drinking water sources. Tartan Group is exempt from Proposition 65 requirements as our business has fewer than 10 employees, however, we feel that it is important to warn CA residents of the possibility that some of our products may contain chemicals that cause cancer, birth defects or other reproductive harm.
More Specific Information about Businesses and Proposition 65:
https://oehha.ca.gov/proposition-65/businesses-and-proposition-65
Proposition 65 also prohibits California businesses from knowingly discharging significant amounts of listed chemicals into sources of drinking water.
Proposition 65 requires California to publish a list of chemicals known to cause cancer, birth defects or other reproductive harm. This list, which must be updated at least once a year, has grown to include approximately 900 chemicals since it was first published in 1987.
Proposition 65 became law in November 1986, when California voters approved it by a 63-37 percent margin. The official name of Proposition 65 is the Safe Drinking Water and Toxic Enforcement Act of 1986.
For More General Information About Proposition 65, please click the link below: